consultancies

MARTIN FLEISCHER
Life science manager
University degree: Italian «Laurea» (comparable to bachelor) in chemistry from Milan University
Citizenship: German
Address: Italia – Via Beato Lodovico Pavoni, 4 – 25050 Provaglio d’Iseo (BS)
DOB: 14 May 1971
Mobile: +393409225430
E-mail: work@martin-fleischer.com
Languages:
working experience
Timeline
February 2023
Freelance consultant
since spring 2019
Services to companies operating in the health care sector:
- set up and fine-tuning of procedures, with a focus on interception and compliance monitoring of transfers of value (ToV) to health care professionals (HCPs) and health care organizations (HCOs)
- decentralization of compliance monitoring by disaggregating legal requirements into independent checklists assigned to different roles within the company
- pre-softwarization audits and assessments
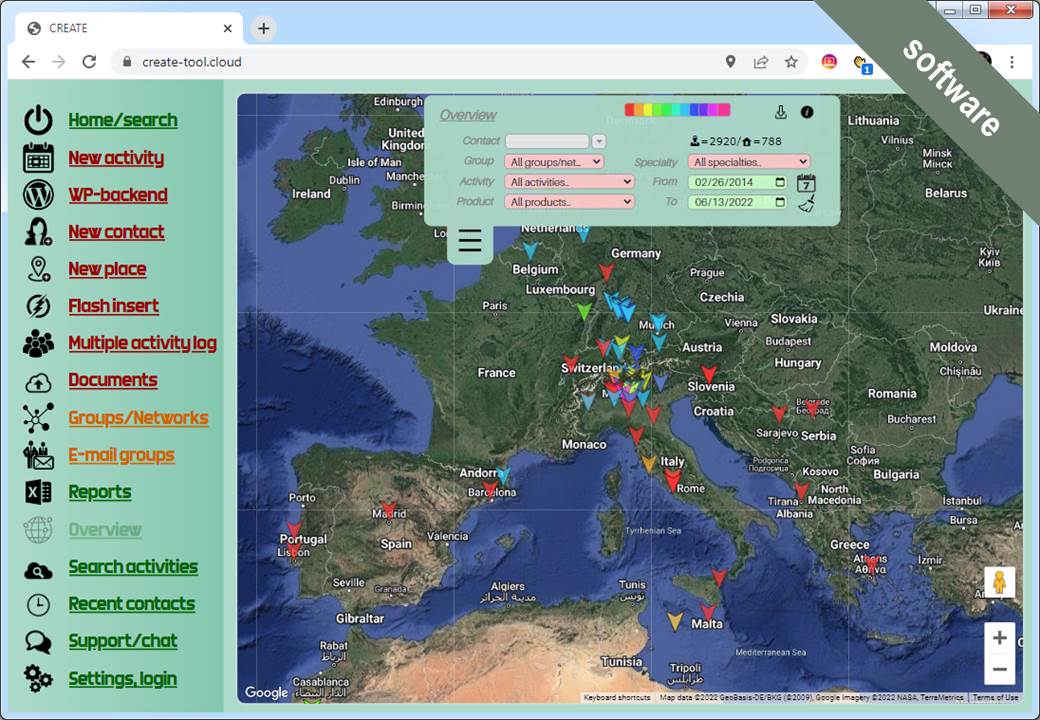
- small custom software solutions based on my CREATE platform
February 2018
HBO (Head of Business Operations) South Europe
Biotest, Via Leonardo da Vinci, 43 – 20090 Trezzano Sul Naviglio (MI)
(from 01/09/2017 to 06/02/2018 = 5 months)
In the TAs (Therapeutic Areas) «Organ Transplantation & Infectology»:
- management of 7 people with scientific and marketing tasks for the Southern Europe region, an area that spans several Mediterranean countries from Portugal to Israel
- management of B2B relationships (both with affiliates and external partner companies)
- development of strategies and tools for objective prioritization of medical projects
- “first time ever” for this German company: development of a bi-lingual contract with an Italian healthcare organization (HCO) to support a nonprofit study in Italy in light of law 17/12/2004 (IgM-FAT trial)
August 2017
Medical Manager
Biotest, Via Leonardo da Vinci, 43 – 20090 Trezzano Sul Naviglio (MI)
(from 01/07/2014 to 31/08/2017 = 3 years and 2 months)
In the TA (Therapeutic Areas) «Organ Transplantation & Infectology»:
usual spectrum of activities of a medical department
- training
- marketing support
- clinical trials support
- relationships with key opinion leaders (KOLs)
- ad-boards
Management of staff (2 MSL = Medical Scientific Liaison), with tasks like
- organization of HSMs (Hospital Staff Meetings) = in-hospital scientific meetings with health care professionals (HCPs)
- management of MQs (Medical Queries) = requests for information from health care professionals (HCPs)
- scientific support & training commercial field force
NOTE: to manage the newly founded medical department, I coded and set-up a GPS-assisted mobile CRM-system. This web application was mainly used to log Hospital Staff meetings (HSMs), FTF-visits with HCPs (Health Care Professionals) and to build a centralized repository of medical information provided by the company, required for quality assurance and pharmacovigilance
June 2014
Medical Manager
Swedish Orphan Biovitrum, Strada Quarta 6/1 D – 43123 Parma (PR)
(from 01/04/2013 to 30/06/2014 = 1 year and 3 months)
In the TAs (Therapeutic Areas) «inflammation/rheumatology»:
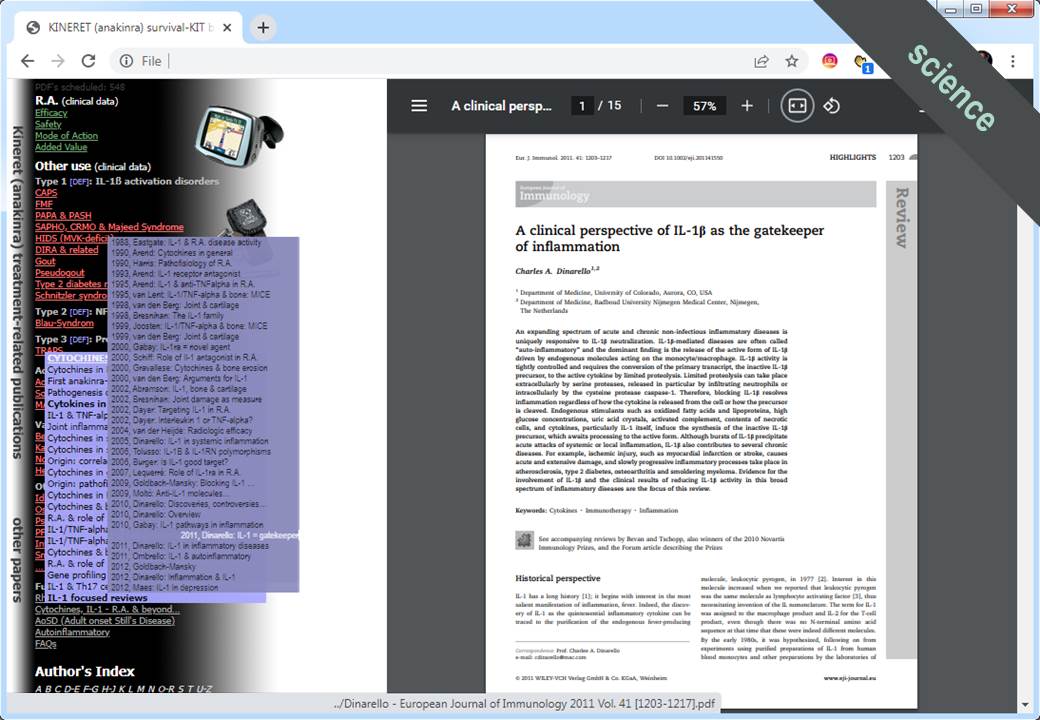
- preparation of the Italian pricing-dossier for the new, EMA-approved orphan indication of the Interleukin-1 receptor antagonist (IL-1Ra) KINERET® (anakinra), concerning the treatment of CAPS (Cryopyrin-Associated Periodic Syndrome), a rare autoinflammatory disorder
- support of Investigator Sponsored Trials (ISS) in accordance with Italian D.M. 17/12/2004 (AIR-TRIP, TRACK)
- development and continuous updating with the latest scientific publications of a literature database compatible with Mendeley®, Papership® and Endnote® citation managers
- organization of HSMs (Hospital Staff Meetings) = in-hospital scientific meetings with health care professionals (HCPs)
- management of MQs (Medical Queries) = requests for information from health care professionals (HCPs)
- collaboration with international task-forces
- marketing support for product strategy
- scientific support & training commercial field force
March 2013
Field Product Manager
Swedish Orphan International, Strada Quarta 6/1 D – 43123 Parma (PR)
(from 23/02/2009 to 31/03/2013 = 4 years and one month)
In the TA (Therapeutic Area) «inflammation/rheumatology»:
Marketing strategy development (and implementation in collaboration with international business partners) for the brand KINERET™ (anakinra), a recombinant human Interleukin-1 receptor antagonist (IL-1Ra), initially approved for the treatment of Rheumatoid arthritis, but due to its striking effectiveness also frequently used off-label for the treatment of IL-1 mediated, autoinflammatory diseases, such as CAPS (Cryopyrin-Associated Periodic Syndrome); TRAPS (Tumor Necrosis Factor Receptor Associated Periodic Syndrome); SJIA (Systemic Juvenile Idiopathic Arthritis); AoSD (Adult onset Still’s Disease) – KINERET™ (anakinra) later gathered approval for the treatment of some these disorders
February 2009
Product Specialist
sanofi-aventis, Viale Luigi Bodio, 37/b – 20158 Milano (MI)
(from 01/01/2007 to 19/02/2009 = 2 years and 2 months)
In the TA (Therapeutic Area) «oncology»:
product specialist in 3 provinces of Northern Italy (Brescia, Trento and Bolzano, because of my native language) for the brand TAXOTERE™ (docetaxel), an antineoplastic drug (cancer drug) approved and used for the treatment of a variety of solid tumors; my task was to provide information regarding its use in the treatment of early and advanced breast cancer to Health Care Professionals (HCPs); during this activity:
- organization of HSMs (Hospital Staff Meetings) = in-hospital scientific meetings with health care professionals (HCPs)
- development and partial writing in cooperation with an oncologist of a pocket guide on the systemic treatment of early-stage breast cancer («Il Taccuino Blu» [in Italian])
December 2006
Product Manager
sanofi-aventis, Viale Luigi Bodio, 37/b – 20158 Milano (MI)
(from 01/01/2005 to 31/12/2006 = 2 years)
In the TA (Therapeutic Area) «oncology»:
- marketing strategy development (and tactical implementation) for the brand ELOXATIN™ (oxaliplatin), an antineoplastic drug (anti-cancer medicine) used for the treatment of colorectal cancer (approved indication) and other types of gastrointestinal cancers
- post-patent strategy for the brand name ELOXATIN™ (oxaliplatin)
- indication management of TAXOTERE™ (docetaxel) for the treatment of advanced gastric cancer
December 2004
Product Specialist
Sanofi-Synthélabo, Via Messina, 38 – 20154 Milano (MI)
(from 01/04/2003 to 31/12/2004 = 2 years and 9 months)
In the TA (Therapeutic Area) «oncology»:
product specialist in 3 provinces in Northern Italy (Brescia, Trento and Bolzano, due to my mother tongue) for the brand ELOXATIN™ (oxaliplatin), an antineoplastic drug (anti-cancer medicine) used for the treatment of colorectal cancer (approved indication) and other types of gastrointestinal cancers
March 2003
Sales Rep (pharma)
Sanofi-Synthélabo, Via Messina, 38 – 20154 Milano (MI)
(from 18/03/2002 to 31/03/2003 = 1 year)
In the TA (Therapeutic Area) «cardiology, urology»:
Sales Rep in the Province of Brescia (Northern Italy)
March 2002
Sales Rep (pharma)
Malesci, Via Lungo L’Ema, 7 – 50012 Bagno a Ripoli (FI)
(from 05/03/2001 to 15/03/2002 = 1 year)
In the TA (Therapeutic Area) «general practitioner»:
Sales Rep in the Province of Brescia (Northern Italy)
Given the corporate demand for high «average daily office visits» I had developed my own CRM that optimized routes, taking into account also availability of general practitioners in peripheral medical offices
Generic skills
Technical skills
legal & regulatory aspects of support for clinical trials
pharmaceutical business & marketing
compliance, application of laws & ethics codes
literature management & use of citation managers
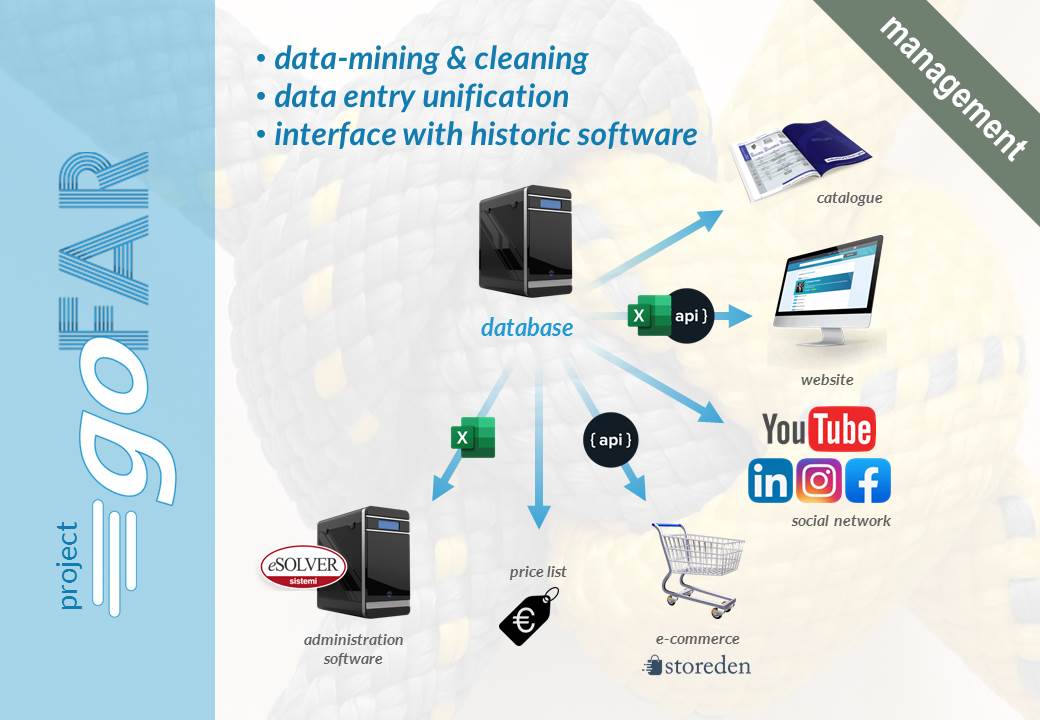
database-design and administration
coding in VBA/VBScript, php, javascript, use of mySQL
design and softwarization of complex procedures
clinical trial design and management
medical writing
regulatory dossier writing
Past Projects
What people say about me
Go ahead – contact me:
* compulsory fields